Adsorption DefinitionAdsorption is a process in which a substance builds up on a surface in increasing concentrations of specific molecular species. When it comes to hydrogen, nitrates, and oxygen, activated charcoal is where these gases adsorb. We must also keep in mind that adsorption and absorption are two distinct processes. The mechanisms used in the two procedures are completely different. 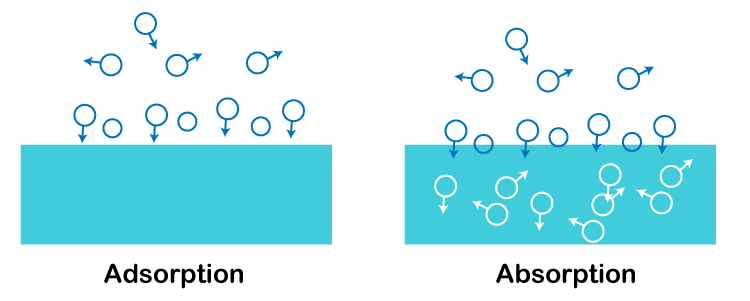 There are two components necessary for the adsorption process: Adsorbate: A material that adheres to another substance's surface. For instance, the gases H2, N2, and O2. Adsorbent: Adsorbate adsorbs on the surface of a substance known as an adsorbent. Charcoal, silica gel, and alumina are a few examples. Different types of AdsorptionAdsorption is divided into following two categories based on the forces that interact between the adsorbent and the adsorbate: Physical adsorption: Physisorption is another name for this kind of adsorption. Weak Van der Waals forces between adsorbent and adsorbate are reason behind physical adsorption. For instance, gases like H2 and N2 adsorb on coconut charcoal. Chemical adsorption: Another name for this kind of adsorption is chemisorption. Powerful chemical forces of the bonding kind between the adsorbent and the adsorbate cause it. We can use the example of iron that forms iron nitride on its surface when heated to 623 K in N2 gas. A spontaneous exothermic reaction is the adsorption of a gas onto a material. The term "heat of adsorption" refers to the energy released when a unit mass of a gas is adsorbed on a surface. Characterstics of AdsorptionPhysical adsorption characteristics:
Chemical adsorption characteristics:
Uses of Adsorption1) Air pollution masks: They are made of silica gel or powdered activated charcoal, and when dust or smoke passes through them, the particles become adsorbent on their surface. 2) Noble gas separation using the Dewar's flask method: In heated coconut charcoal, a mixture of the noble gases Ne, Ar, and Kr is transferred through a Dewar flask. Neon remains after Argon and Krypton have been adsorbed. 3) Water purification: Impurities are adsorbed on the alum and removed from the water by the addition of an alum stone. 4) Elimination of humidity and moisture: Silica gel absorbs water molecules and removes moisture from the air. 5) Adsorption chromatography: Hormones and pigments are separated using this method. 6) Ion exchange technique: Calcium and magnesium ions are adsorbed on the surface of the ion exchange resin in this process of softening water. 7) In metalworking: The particle is adsorbed on the froth during the concentration of ore by froth flotation process. Various Instances of AdsorptionAdsorption of water Adsorption of water at surfaces plays a significant role in chemical engineering, materials science, and even catalysis. When water is present on the surfaces of solids due to physical or chemical adsorption, it is referred to as surface hydration. In a variety of systems, this plays a crucial part in regulating interface properties, chemical reaction routes, and catalytic efficacy. Surface hydration is typically abolished in the case of physically adsorbed water by the drying process, which takes place at conditions of temperature and pressure and furthers the complete vaporization of water. Regarding water that has been chemically adsorbed, hydration might take the form of dissociative adsorption or molecular adsorption. Virus Adsorption Adsorption comes first in the viral life cycle, then penetration, uncoating, synthesis, and release. The virus replication cycle is often the same for various virus types. Adsorption of Polymers The surfaces of polymers exhibit molecular adsorption. These qualities are crucial for a variety of use-case scenarios. For instance, it's crucial for the creation of non-stick coatings and a number of biomedical equipment. Polymers may also be adsorbed to surfaces using the polyelectrolyte adsorption process. Catalyst adsorption There is typically an acceleration of particular chemical reactions when adsorption on molecules occurs on some catalytic materials. Adsorption IsothermIsotherms are frequently used to characterize adsorption. It is because temperature affects the entire process greatly or has a significant part in both. Furthermore, the adsorption method is described using a number of isotherm models. Freundlich TheoryWhen the adsorbate forms a monomolecular layer on the surface of the adsorbent, the adsorption obeys the Freundlich adsorption isotherm. The Freundlich adsorption isotherm's major flaw is that it breaks down at high pressure. The process of multi-layered adsorption could not be explained by it. Langmuir TheoryIn 1916, Langmuir presented that a solid's surface may be divided into elementary sites, each of which could adsorb a single gas. The ability of a gas molecule to bind to any one site is presumed to be independent of whether or not the neighbouring sites are occupied, and all adsorption sites are thought to be equivalent. It is also assumed that there is a dynamic equilibrium between gas molecules that have been adsorbed and those that have not. These guidelines can be drawn from the Langmuir adsorption isotherm:
BET TheoryIn 1938, Brunauer, Emmett, and Teller proposed the BET theory. According to this idea, multilayer adsorption forms during physisorption. This theory also discusses the homogeneity of solid surface adsorption sites. It is assumed that when adsorption takes place at one site, it won't have an impact on adsorption at nearby locations. Difference between Adsorption and Absorption
Next TopicCapacitance Definition |