Solution DefinitionIntroductionA solution is a specific kind of homogenous combination made up of two or more components that are used in chemistry. A solute is a material that has been dissolved in a solvent, which is the other component in the combination. The solvent particles will pull the solute particles apart & surround them if the pulling forces between the solvent & solute particles are stronger than the attractive forces keeping the solute particles together. The solute particles enclosed by the solid solute subsequently disperse into the solution. Chemical polarity effects are engaged in mixing a solution at a scale that leads to interactions unique to solvation. When the solvent makes up a significant portion of the combination, as is often the case, the solution typically has the condition of the solvent. One crucial parameter is the concentration of a solution, which measures how much solute is present in a certain volume of solution or solvent. When water is one of the solvents, the phrase "aqueous solution" is used.  Characteristics of Solution
Types of SolutionWhen a combination is homogeneous, its constituent parts come together to create a single phase. The term "heterogeneous" refers to a combination whose constituent parts are of various phases. The mixture's characteristics (such as temperature, concentration, & density) may be dispersed evenly across the volume, but only in the absence of or after the conclusion of diffusion processes. The component that is present in the highest quantity is often thought of as the solvent. Solids, liquids, or gases may all be solvents. Solutes are any one or more elements in the solution apart from the solvent. The solvent and the solution are in the same physical state. 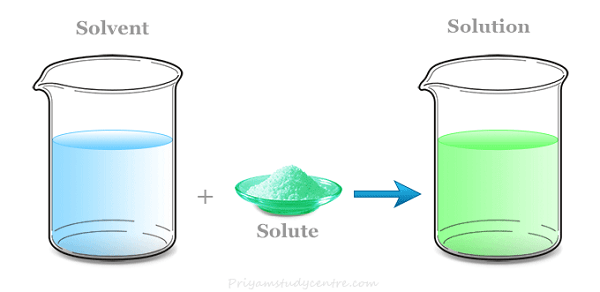 Gaseous mixtureOnly gas (non-condensable) or vapors (condensable) are dissolved in the case of a gaseous solvent under a certain set of circumstances. Air, which has nitrogen along with other gases dissolved in it, is an example of a gaseous solution. Non-condensable gases create fairly simple solutions because interactions among gaseous molecules are essentially irrelevant. They are simply referred to as homogenous mixes of gases in the literature and are not even categorized as solutions. The homogeneity of the gaseous systems is guaranteed by the Brownian motion and the constant molecular disturbance of gas molecules. In contrast to condensable gas mixes, non-condensable gas mixtures (such as air/CO2 or air) are unable to naturally separate into discrete stratified layers as an indication of relative density. Under typical Earthly circumstances, diffusion forces effectively offset gravitational forces. Contrarily, in the situation of condensable vapors, the surplus vapor condenses into liquid form once the saturation vapor pressure at a certain temperature is attained. 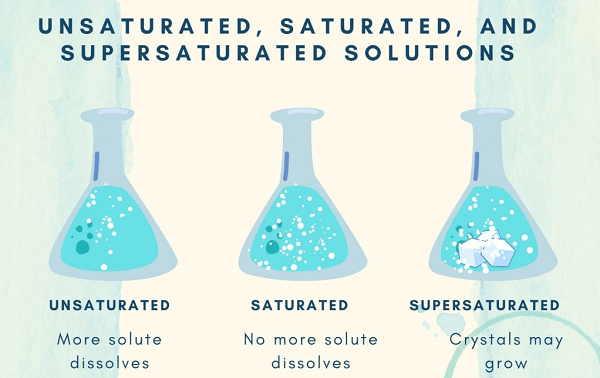 Liquid SolutionsAlmost all solids, gases, and liquids can be dissolved if a solvent is a liquid. Here are a few instances: 1. Gas in a liquid: Carbon dioxide in water is a less straightforward example since a chemical reaction (the production of ions) occurs along with the solution. The dissolved gas itself is not visible because it is dissolved at the molecular level; the visible bubbles in carbonated water are actually an effervescence of the gas carbon dioxide that emerged out of the solution. 2. The liquid in liquid: The creation of a constant by combining two or more compounds with the same chemical reactions but varying concentrations. (Solution homogenization) Alcoholic drinks are essentially ethanol in water solutions. 3. In a liquid, a solid
 Solid solutionsGases, liquids, & solids may all dissolve if the solvent is solid.
SolubilitySolubility refers to a substance's capacity to dissolve into another substance. Both liquids are said to be miscible when one can entirely dissolve the other. It is claimed that two substances are immiscible if they can never combine to produce a solution. All solutions have positive mixing entropies. There may or may not be an energy preference for the interactions between certain molecules or ions. The free energy falls with increasing concentrations of solutes if interactions are unfavorable. The solution is considered to be saturated when no more of the solute particle can be dissolved since the energy loss eventually surpasses the entropy gain. However, depending on many environmental elements like temperature, pressure, and pollution, the point at which a solution might become saturated can vary dramatically. For certain mixtures of solute and solvent, a supersaturated solution may be created by first increasing the solubility to dissolve more solute (for instance, by raising the temperature) and then reducing it (for instance, by cooling). Typically, a solvent may dissolve more of a particular solid solute at a given temperature. However, the solubilities of most gases and certain chemicals show a temperature-dependent decline. The exothermic enthalpy of the solution is what causes this behavior. Some surfactants behave in this way. Compared to solids or gases, the solubility of liquid in other liquids is often less temperature-sensitive. PropertiesWhen new compounds are introduced, the physical characteristics of the compounds, such as their melting and boiling points, alter. Collaborative qualities describe them all together. The quantity of one chemical dissolved in other substances is referred to as concentration and may be measured in a variety of methods. Molarity, volume fraction, & mole fraction are a few examples. Liquid Solution CharacteristicsFluid noble gases, molten metals, molten salts, molten covalent networks, & molecular liquids may all act as solvents in theory. The majority of solvents used in chemistry and biochemistry are molecular liquids. Depending on whether their molecules have a persistent electric dipole moment, they may be divided into both polar and non-polar groups. Another difference between protic & aprotic solvents is whether or not their molecules are capable of creating hydrogen bonds. The most popular solvent, water, is polar and may support hydrogen bonding. Since water molecules can establish hydrogen bonds and are polar, they make an excellent solvent. In polar solvents, salts dissolve, creating both negative and positive ions that are drawn to the opposite ends of the solvent molecule. Hydration happens when the charged ions of the solute are encircled by water molecules when water is the solvent. A common example is aqueous salt. Electrolytes are such solutions. Ion association has to be given consideration whenever salt dissolves in water. When polar solutes are dissolved in polar solvents, polar or hydrogen bonds are created. For instance, ethanol is an aqueous solution and is present in all alcoholic drinks. In contrast, non-polar solvents facilitate the better dissolution of non-polar solutes. Examples include hydrocarbons, which mix well but are incompatible with water, such as oil and grease. The oil leaking from a damaged ship doesn't dissolve into the ocean water and instead floats on the surface, demonstrating the immiscibility of water and oil. ConclusionIn this article, we have learned about what is solution, its definition, and its various types. It is a type of homogeneous mixture in which solute is dissolved in solvent. Next TopicSpore Formation Definition |