BoilingBoiling is a state to bring the liquid into the vaporization form. A liquid is heated till it reaches the vaporization state, known as the boiling state of that liquid. The temperature, at which the boiling state of the liquid is achieved, is known as the boiling point of the liquid. Impact of boiling on the three states of matterWe have discussed the impact of boiling on the liquid. We commonly use the term boiling with the liquid only. But, what about the other states of the matter? And what will be the impact of boiling on the solid and gas? Boiling point is generally defined as the temperature at which the substance's vapor pressure becomes equal to the atmospheric pressure. Let's discuss this in detail. SolidSolid is the substance in which the atoms are tightly bound to each other. We can also say that solids have a high force of attraction. Metal has positive and negative ions between them, and it constitutes the electrostatic force that keeps the atoms of the solid strongly packed. Thus, the higher the energy required by a substance, the higher is the boiling point. The solid is heated in a furnace with very high temperatures. The metal first starts melting, which is its liquid state. At this point, the melting point is calculated when it melts completely. The heating continues till the melted metal starts vaporizing. When the metal starts vaporizing, the boiling point is calculated. We can also say that the state of a metal when its pressure is achieved is the boiling point. Hence, the boiling points of the solids are generally very high. Note: The vaporizing state is the transition of a substance from the liquid state to the vapor state.LiquidThe liquids do not have a melting point because it is already present in their liquid state. But, it varies in the case of liquids at different temperatures. The liquid is heated until it reaches the vaporizing state. But, sometimes, these liquids can become solid at low temperatures. The boiling point of liquids is low compared to solids because the atoms in the liquids are loosely packed. Thus, the boiling point of a liquid is defined as the temperature at which a liquid reaches its vaporization state. For example, The boiling point of benzene is 80 degrees Celsius, and benzene is liquid at room temperature. GasesThe boiling point of gases is very less as compared to solids and gases. It is because gases are already present in the gaseous state. The atoms in the gas are very loosely packed. It takes a little heat to convert a gas into its vaporization state. For example, The boiling point of carbon disulfide is 46.2 degrees Celsius. Since carbon dioxide and oxygen are already in the vaporization state in the atmosphere, their boiling point is -78.5 degrees Celsius and -183 degrees Celsius. Types of Boiling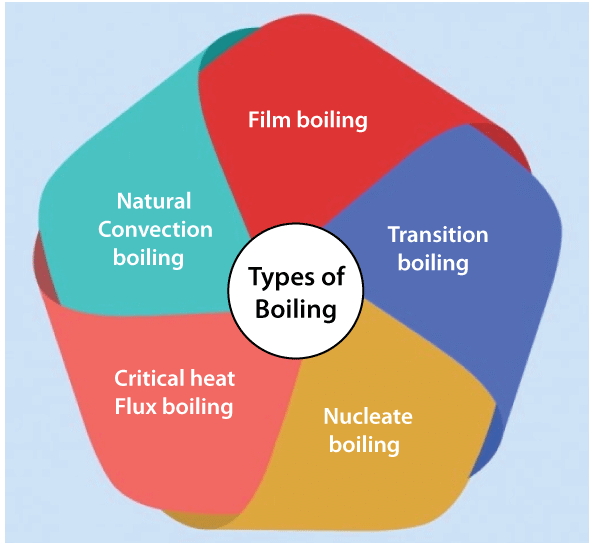
Let's discuss in detail. Natural Convection boilingThe general requirement of a substance to be boiled is that the temperature at the walls of the container should be equal to the saturation temperature. But practically, boiling occurs when the liquid is heated above the saturation temperature. The vapor formation also occurs when the surface temperature is greater than the saturation temperature. 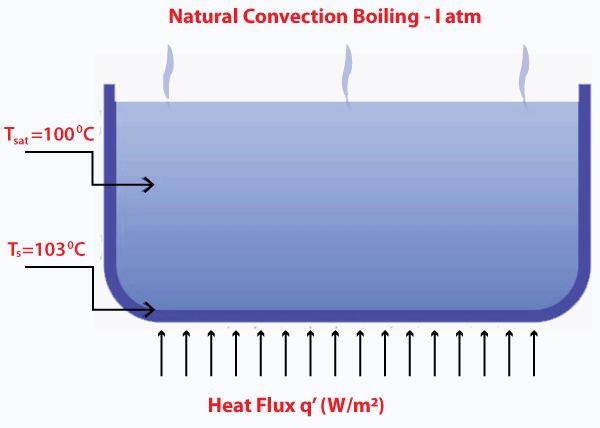
The general requirement for the natural convection boiling is given by: Saturation temperature < 5 degrees Celsius Nucleate boilingIt is the most common type of boiling. The temperature of heat is generally high at the surface as compared to the saturated fluid temperature. The nucleate term refers to the formation of bubbles, which carry the heat flux from the surface to the main stream of the water. It enhances the heat transfer in the boiling process. The formation of these bubbles look likes the image shown below: 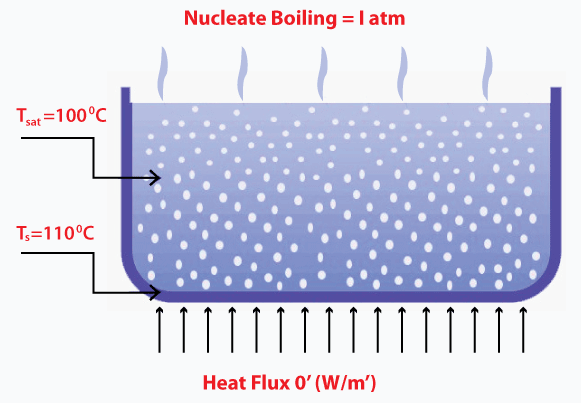
As soon as the bubble reaches the main fluid stream, it collapses. The temperature at the fluid stream is not as high as the surface where bubbles were initially created, and the energy in this process is effectively carried away. Film boilingAs discussed, bubbles form at a specific temperature, allowing the heat transfer from the surface to the mainstream of the water. The increase in temperature causes the formation of vapor film on the surface. The temperature at the surface is much higher than the temperature of the liquid, as shown below: 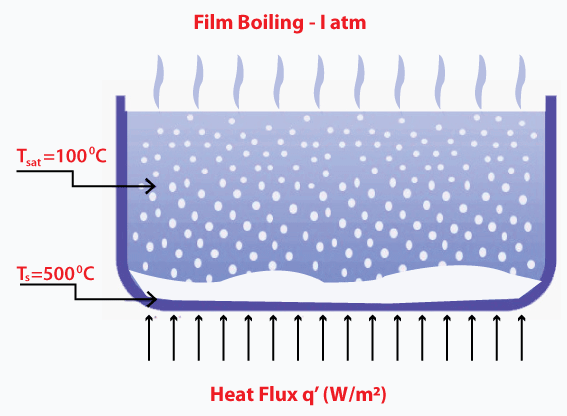
The formed vapor layer has lower heat transferability. The material carrying out this process should be strong enough to withstand the temperature at the surface. Otherwise, it can harm the material. Such a process is known as burnout. Transition boilingThe steam is produced during the boiling process from a vapor layer over the surface of the liquid, known as the insulating vapor layer. It adversely affects the heat flux because this layer covers a large portion of the surface. Thus, at this point, the transition boiling becomes unstable. 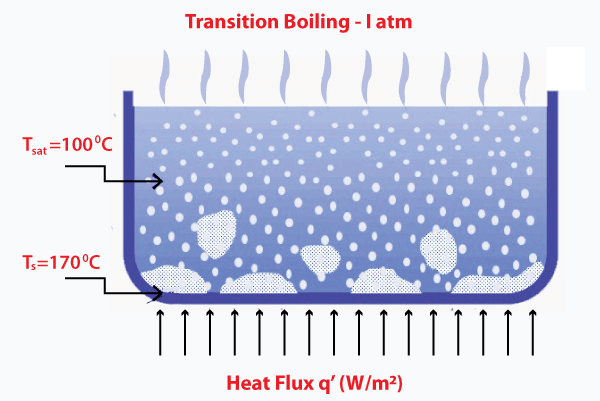
Boiling crisis: The transition from nucleate boiling to film boiling is known as boiling crisis. Critical heat Flux boilingThe CHF or Critical Heat Flux is a type of boiling where a phase change occurs. The heat transfer in the above nucleate boiling cannot be increased. Sometimes, it is known as critical heat flux. A vapor film is created on the surface of the liquid when the temperature rises above the critical temperature. At this temperature, bubbles do not form. The vapor layer also decreases the efficiency of the heat transfer. At this point, the temperature on the surface of the liquid rises very rapidly. Contrast of boiling with other termsLet's discuss the comparison of boiling with melting, steaming, and evaporation. It will help to figure out the differences between these terms. 1. Boiling vs. MeltingAs the name implies, boiling means to boil and melting means to melt. When a substance changes its state from solid to liquid, it is termed as melting of that solid. When a substance changes its state from liquid to gas, it is termed as boiling of that liquid. Thus, we can say that melting is generally concerned with the solids and boiling with liquids. 
The melting and boiling point of different substances are different. For example, the melting point of Iron is 1538 degrees Celsius, while its boiling point is 2862 degrees Celsius. 2. Steaming vs. Boiling
Steaming means to provide steam generated after the liquid is boiled. Steam has the highest amount of heat energy, and it is because of the additional latent heat of vaporization. The steaming process is generally used in cooking to preserve the color, nutrients, and flavor of food. The items to be steamed are kept away from the contact of the water. It is a type of moist heat. Boiling is a process where the items are kept submerged in the water, and it is preferred in the case of large-scale cooking. 3. Boiling vs. EvaporationEvaporation is defined as the process of converting the liquid state into vapors below the boiling point of that liquid. It is a slower process and occurs only from the surface of the liquid, and it does not produce any amount of bubbles during the process. Boiling is defined as the process of converting the liquid state into the vapors to the boiling point of that liquid. It is a faster process and occurs throughout the liquid, and it also produces bubbles during the process. Applications of BoilingThe applications of boiling are as follows:
Advantages of boilingThe advantages of boiling are as follows:
Disadvantages of boilingThe disadvantages of boiling are as follows:
To prevent the loss of nutrients in food, it is recommended to boil the vegetables, pulses, etc., in less amount of water, or the left over water should not be drained off. It can be consumed in different ways. Some items, such as beetroot, should be boiled with the peel to prevent the color loss.
Next TopicCarnot Engine
|
 For Videos Join Our Youtube Channel: Join Now
For Videos Join Our Youtube Channel: Join Now
Feedback
- Send your Feedback to [email protected]
Help Others, Please Share









