Unit of electric chargeCoulomb The S.I. (System of International) unit of electric charge is Coulomb. One coulomb is defined as the amount of electric charge per unit. The electric charge e is equal to 1.602 x 10-19 C. 1 e = 1.602 x 10-19 C So, 1C = 1/1.602 x 10-19 Or 1 C = 1/e It is represented by single uppercase alphabet C. Thus, one coulomb is equal to the 1/1.602 x 10-19 elementary charges. Both the charge and elementary charge have the same magnitude. But, the sign (negative or positive) is the opposite. It is equal to -1 C.
According to the International system of Base Units, one coulomb is defined as the charge passing through the conductor carrying current one amperes in one second. 1 C = 1A. 1s C = A.s Amperes is the unit of current and seconds is the unit of time. What is an electric charge?The electric charge is a physical quantity that experiences a force when placed in an electromagnetic field. A charge can be neutral, positive, or neutral. Proton and electrons are the positive and negative charges of an atom. The neutron does have any charge and is thus neutral. The electron and protons are the charges with the same magnitude but opposite signs. The charge on an electron is -1.602 x 10-19C, while the proton is 1.602 x 10-19 C. 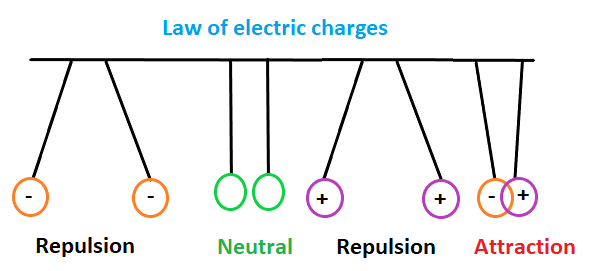
The same charges repel each other (force or repulsion between the two) and the opposite charges attract each other (force of attraction between the two). In electrical circuit, the atoms carrying negative charges are often termed as electrons, and the atoms carrying positive charges are known as holes. The unit of charge in electrical is defined as C = Ah (Ampere-hour). Electric charge formulasLet's discuss some of the formulas of electric charge. Q = n e Where, n = No. of electrons e = charge of an electron Q is the charge in coulomb Q = I t Where, I is the current is Amperes t is the time in seconds or hour Q is the charge in coulomb Q = CV Where, Q is the charge in coulomb C is the capacitance in Farads Vis the voltage in volts 1 mole of charge = 6.023 x 1023 (Avogadro's number) 1 Farad = 96500 Coulombs Multiples of CoulombThe unit of charge, i.e., coulomb, is also expressed in the multiples. Some of the values are listed as follows:
Other S.I unitsApart from electric charge, there are S.I units of various electrical parameters. It is shown in the below table.s
Numerical ExamplesLet's discuss some Numerical Examples of electric charge. Example 1: A current of 10 Amperes flows in a circuit. What time does it take for 1000 C to pass through the circuit? Solution: Given: I = 10 A and Q = 1000 Coulombs I = Q/T Current = Charge/Time Or T = Q/I T = 1000/10 T = 100 seconds Thus, the time requires for the charge to pass through the circuit is 100 seconds. Example 2: A container contains 400g of water. Find the positive charge present in that container. Solution: The formula to calculate the total charge is given by: Q = n e Where, n = No. of electrons e = charge of an e- Given: Water in the container = 400 g Mass of water = 18 g/mol 1 mol = 6.023 x 1023 18 g/mol = 6.023 x 1023 No. of molecules in 400g of water = 400/18 x 6.023 x 1023 = 133.8 x 1023 Water (H2O) contains 2 atoms of hydrogen and 1 atom of oxygen. So, the number of electrons equals the sum of electrons in the hydrogen atom and oxygen atom. The hydrogen contains 2 electrons, and oxygen contains 8. So, total electrons = 8 + 2 = 10 Total charge = Q = n e Q = 133.8 x 1023x 10 x 1.602 x 10-19 Q = 214.08 x 105 Q = 21.408 x 106 Or Q = 21.408 Megacoulomb Thus, the total positive charge present in the container is 21.408 x 106 Coulombs.
Next TopicSolar Energy
|
 For Videos Join Our Youtube Channel: Join Now
For Videos Join Our Youtube Channel: Join Now
Feedback
- Send your Feedback to [email protected]
Help Others, Please Share









