What is Quantum Mechanics?The physical features of nature at the size of atoms and subatomic particles are described by the fundamental physics theory known as quantum mechanics. It serves as the theoretical cornerstone for all branches of quantum physics, including quantum information science, quantum technology, quantum field theory, and quantum chemistry. Many parts of nature are described by classical physics, a body of theories that predated the development of quantum mechanics, at a large (macroscopic) scale, but not well enough at microscopic (atomic and subatomic) sizes. Most classical physics theories can be derived from quantum mechanics as a large-scale (macroscopic) approximation. With respect to energy, momentum, angular momentum, and other quantities of a bound system, quantum mechanics differs from classical physics in that these quantities are constrained to discrete values (quantization), objects exhibit wave-like and particle-like properties (wave-particle duality), and the uncertainty principle places restrictions on how accurately physical quantities can be predicted before being measured. Progressively, theories to explain observations that could not be explained by classical physics, such as Max Planck's solution to the black-body radiation problem in 1900 and Albert Einstein's 1905 paper explaining the photoelectric effect, led to the development of quantum mechanics. These early investigations into microscopic phenomena?now referred to as the "old quantum theory"?led to the complete formulation of quantum mechanics by Niels Bohr, Erwin Schr�dinger, Werner Heisenberg, Max Born, Paul Dirac, and others in the middle of the 1920s. Modern theory is expressed in several newly created mathematical formalisms. One of them describes what measurements of a particle's energy, momentum, and other physical parameters may reveal in terms of probability amplitudes. This mathematical entity is known as the wave function. In a publication, the photoelectric effect was explained. Summary and foundational ideasCalculating the characteristics and behavior of physical systems is made possible by quantum mechanics. Molecular, atomic, and subatomic systems are the typical targets of its application. Although its applicability to people involves philosophical issues, such as Wigner's friend, and its applicability to the cosmos is still hypothetical, it has been shown to hold for complex molecules with thousands of atoms. Experimentally, quantum mechanical predictions have been extremely accurately confirmed. A key aspect of the theory is that it typically only provides probabilities rather than exact predictions of what will happen. The square of a complex number's absolute value, or probability amplitude, is used to calculate probabilities in mathematics. A key aspect of the theory is that it typically only provides probabilities rather than exact predictions of what will happen. The square of a complex number's absolute value, or probability amplitude, is used to calculate probabilities in mathematics. In honor of physicist Max Born, this is referred to as the Born rule. For instance, a wave function that assigns a probability amplitude to each location in space can be used to describe a quantum particle like an electron. When the Born rule is applied to these amplitudes, a probability density function for the position of the electron in the experiment to measure it is produced. The theory can only accomplish so much; it cannot predict with certainty where the electron will be discovered. The Schr�dinger equation establishes a connection between the collection of probability amplitudes that correspond to one point in time and the collection that relates to another. The most well-known version of this uncertainty principle states that it is impossible to make an exact forecast for a measurement of a quantum particle's position and momentum at the same time, regardless of how thoroughly experiments are planned or how carefully a quantum particle is prepared. The phenomenon of quantum interference, which is frequently demonstrated using the double-slit experiment, is another effect of the mathematical principles of quantum mechanics. In the simplest form of this experiment, a plate with two parallel slits across it is illuminated by a coherent light source, like a laser beam, and the light passing through the slits is seen on a screen behind the plate. The interference between the light waves travelling through the two slits caused by light's wave nature results in bright and dark bands on the screen, an unexpected outcome if light were made up of classical particles. The interference pattern is shown by the varied densities of these particle strikes on the screen, as opposed to the fact that light is always observed to be absorbed at the screen at discrete spots as individual particles rather than waves. Additionally, variants of the experiment with detectors at the slits discover that each photon is observed passing through one slit (as a classical particle would) rather than both slits (as a wave would) as would be the case with a wave. These tests show, however, that if one can determine which slit particles flow through, they do not create an interference pattern. The same behaviour is discovered in other atomic-scale objects, such electrons, when they are fired at a double slit. Wave-particle duality is the name given to this behaviour. A particle that comes up against a potential barrier can cross it even if its kinetic energy is less than the maximum of the potential, a counterintuitive occurrence predicted by quantum mechanics. A particle that comes up against a potential barrier can cross it even if its kinetic energy is less than the maximum of the potential, a counterintuitive occurrence predicted by quantum mechanics. This particle would be trapped if classical mechanics applied. In addition to facilitating nuclear fusion in stars and radioactive decay, quantum tunnelling also has crucial uses in the tunnel diode and scanning tunnelling microscopy. Quantum entanglement, which occurs when quantum systems interact, is the result of the features of the systems being too interwoven to be described purely in terms of their component parts. "The characteristic feature of quantum mechanics, the one that enforces its entire departure from classical lines of thought," wrote Erwin Schr�dinger, was entanglement A useful tool in communication protocols like quantum key distribution and superdense coding, quantum entanglement permits the counterintuitive qualities of quantum pseudo-telepathy. Contrary to popular belief, the no-communication theorem shows that entanglement does not permit transmission signals faster than the speed of light. Testing for "hidden variables"?hypothetical features more fundamental than the quantities covered in quantum theory itself?is another avenue made possible by entanglement. If these properties exist, they would enable more precise predictions than those possible with quantum theory. Broad classes of such hidden-variable theories have been shown to be incompatible with quantum physics by several findings, most notably Bell's theorem. The findings of a Bell test will be confined in a specific, quantifiable way if nature genuinely behaves in accordance with any theory of local hidden variables, according to Bell's theorem. Numerous Bell tests involving entangled particles have produced findings that are inconsistent with the limitations imposed by local hidden variables. Formulation in mathematicsThe state of a quantum mechanical system is a vector psi belonging to a (separable) complex Hilbert space H in the mathematically exact formulation of quantum mechanics. It is hypothesized that this vector is normalized under the Hilbert space inner product, obeying (psi,psi) = 1, and that it is well-defined up to a complex number of moduli 1 (the global phase), meaning that psi and e(i?) *psi reflect the same physical system. In other words, the points in the complex projective space, which is another name for the projective space of a Hilbert space, are the potential states. For example, the Hilbert space for describing position and momentum is the space of complex square-integrable functions L^2(C), whereas the Hilbert space for the spin of a single proton is just the space of two-dimensional complex vectors C^2 with the usual inner product. Position, momentum, energy, and spin are examples of physical quantities of interest. These physical quantities are represented by observables, which are Hermitian (or, more accurately, self-adjoint) linear operators acting on the Hilbert space. An observable's eigenvector can be a quantum state, in which case it is referred to as an eigenstate, and the corresponding eigenvalue is equal to the value of the observable in that eigenstate. Thus, measurement is the source of quantum mechanics' probabilistic nature. One of the most challenging characteristics of quantum systems to comprehend is this. It was the main subject of the renowned Bohr-Einstein discussions, in which the two scientists sought to shed light on these underlying ideas through thought experiments. The definition of a "measurement" has been intensively researched in the decades since the development of quantum physics. The idea of "wave function collapse" has been eliminated in more recent interpretations of quantum mechanics (see, for instance, the many-worlds interpretation). The fundamental principle is that when a quantum system interacts with a measurement device, its individual wave functions entangle and the original quantum system loses its independence. See the article on measuring in quantum mechanics for further information. WHAT YEAR DID QUANTUM MECHANICS ARRIVE?According to the University of St. Andrews in Scotland, quantum mechanics was first proposed as a collection of contentious mathematical explanations for phenomena that the mathematics of classical mechanics were unable to explain. It began at the beginning of the 20th century, at the time Albert Einstein published his theory of relativity, a different revolution in physics that explains the motion of objects moving quickly. However, unlike relativity, quantum mechanics cannot be traced back to a single researcher. Instead, numerous scientists contributed to a foundation that, between the late 1800s and 1930, gradually acquired recognition and experimental proof. According to the Perimeter Institute, German physicist Max Planck was attempting to explain why objects at particular temperatures, such as the 1,470°F (800°C) filament of a light bulb, glow a particular color, in this case red. Planck came to the conclusion that the relationship between temperature and color could be explained using the equations developed by physicist Ludwig Boltzmann to describe the behavior of gases. The issue was that Boltzmann's theories assumed that light was also made up of discrete bits because any given gas was composed of small particles. This theory ran counter to prevalent theories at the time regarding light, when most scientists thought that light was a continuous wave rather than a small packet. Even though Planck himself did not believe in atoms or discrete particles of light, his theory gained support in 1905 when Einstein wrote a paper titled "Concerning a Heuristic Point of View Towards the Emission and Transformation of Light." In Einstein's theory, light instead of travelling as a wave travel as some sort of "energy quanta." In his work, Einstein hypothesized that this energy packet could only "be absorbed or generated as a whole," specifically when an atom "jumps" between quantized vibration frequencies. The "quantum" element of quantum mechanics derives from this. WAVE-PARTICLE DUALITY: WHAT IS IT?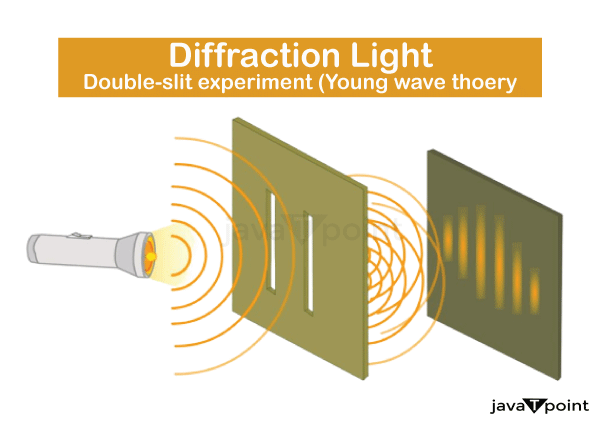
Particles in quantum mechanics can occasionally exist as waves and occasionally as particles The double-slit experiment, in which particles like electrons are fired at a board with two slits cut into it and a screen behind it that illuminates when an electron strikes it, is the most famous example of this. According to a well-known article in Nature, if the electrons were particles, they would have left two bright lines where they had struck the screen after passing through one or the other of the slits. Rather, when the experiment is run, a screen interference pattern emerges.Only if electrons are waves with crests (high points) and troughs (low points) that can interfere with one another does this pattern of dark and brilliant bands make sense. The interference pattern is visible even when only one electron is sent through the slits at once, simulating the effect of an electron interfering with itself. The equations of Einstein's theory of special relativity were utilised by French physicist Louis de Broglie in 1924 to demonstrate that waves and particles can both display wave-like properties. A few years later, he was awarded the Nobel Prize for this discovery. How Do Atoms Get Described By Quantum Mechanics?Niels Bohr, a Danish physicist, attempted to use quantum mechanics in the 1910s to describe the interior structure of atoms. By this time, it was understood that an atom's structure consisted of a small, light, negatively charged electron cloud around a massive, dense, positively charged nucleus. Bohr placed the electrons in orbits around the nucleus that were like planets in a subatomic solar system, with the exception that their orbital distances were strictly limited. The atom may receive or emit radiation at energies by hopping from one orbit to another, demonstrating their quantum nature. The American Physical Society reported that shortly after, two scientists, working independently and utilizing different lines of mathematical reasoning produced a more thorough quantum model of the atom. This was accomplished by German physicist Werner Heisenberg by creating "matrix mechanics." Erwin Schr�dinger, an Austrian-Irish physicist, created a related concept known as "wave mechanics." In 1926, Schr�dinger demonstrated that these two methods were comparable. The previous Bohr model of the atom was replaced by the Heisenberg-Schr�dinger model, in which each electron behaves as a wave around the atom's nucleus. The electrons in the Heisenberg-Schr�dinger model of the atom follow a "wave function" and reside in "orbitals" as opposed to actual orbits. According to a website that explains atomic orbitals, they can take on a variety of shapes, such as spheres, dumbbells, and daisies, in contrast to the Bohr model's circular orbits.
Next Topic#
|
 For Videos Join Our Youtube Channel: Join Now
For Videos Join Our Youtube Channel: Join Now
Feedback
- Send your Feedback to [email protected]
Help Others, Please Share









