Acid RainAcid Rain refers to rain that is abnormally acidic. In simple words, it is the rain that is acidic in nature due to some pollutants like Nitrogen Oxides (NOx) and Sulfur Dioxide (SO2) present in the air. The water vapor on reacting with Nitrogen oxides and Sulphur dioxide produces nitric and sulphuric acid respectively. So, any type of precipitation that contains acidic components like sulphuric or nitric acid and fall to the ground in wet or dry form can be called acid rain. For example, rain, snow, fog, hail, dust, etc. The term acid rain was given by Scottish chemist Robert Angus Smith in 1852. 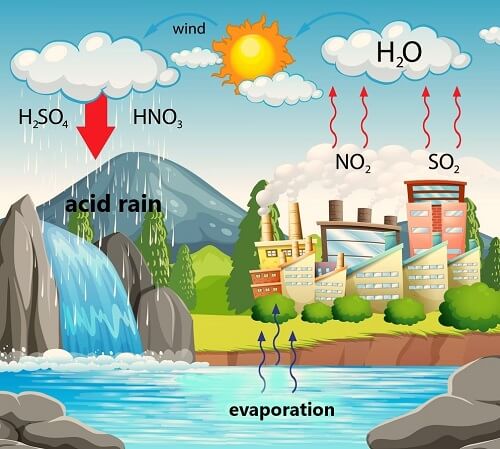
Rain is generally slightly acidic always as it has things dissolved in it naturally. However, when the rain comes in contact or reacts with some air pollutants such as sulfur or nitrogen oxides, it leads to the formation of sulfuric or nitric acid. The acidity of acid rain is on par with that of lemon juice and thus it is more caustic than plain water. The pollutants that cause acid rain are mostly emitted from vehicles, industries, and power-generating plants. In normal rain, the carbon dioxide and water in the air react to form carbonic acid, a weak acid. So, the pH of normal rain, which is slightly acidic ranges from 5.3 to 5.6, whereas, the pH of acidic rain lies between 4.2 and 4.4. What causes acid rain?Acid rain is the outcome of human activities as its main causes are coal-burning power plants, factories and vehicles. Two-thirds of SO2 and one-fourth of NO is released into the air by electric power generators. However, the minor causes include rotting vegetation, erupting volcanoes, etc. The pollution caused by human activities releases sulfur dioxide (SO2) and nitrogen oxides (NO) into the atmosphere. These oxides react with water, oxygen and other substances in the air to form sulfuric and nitric acid. These compounds may be carried by wind to different places through the atmosphere. The acid rain when falls on Earth flows across the surface and enters water bodies and is also absorbed by the soil. Effects of acid rainEffect on water bodies: The acidic rainwater either directly enter the aquatic bodies or falls on the ground first then flows into rivers, lakes, ponds, etc. Thus, acids get accumulated in the water bodies and lower the pH of their water. It affects the lives of aquatic plants and animals as they need a specific pH level of around 4.9 for their survival. Effect on the forest: It tends to damage the leaves of trees thus making them vulnerable to insects, extreme weather and diseases. It also damages the bark and may arrest the growth of trees. Many cases of forest damage due to acid rain have been reported in Germany, Switzerland and Poland. Effect on soil: Acid rain affects the soil chemistry as well as flora and fauna. It damages the soil chemical composition such as soil pH, and soil microbes and biological activity. The hydrogen ions in acid rain also take away minerals and nutrients from soil such as calcium and magnesium. So, due to the low level of nutrients in soil like calcium, potassium, magnesium, etc., the growth of plants and animals may get affected. Effect on vegetation and plantations: The vegetation, and plantation cover that is encircled by acidic fogs and clouds is damaged due to the effects of acids. The long term exposure to acid rain may damage forests and vegetation cover permanently. Effect on building: The acid deposited by acid rain tend to corrode buildings especially those that are made of limestone. It weakens the buildings and makes them susceptible to decay. Besides buildings, cars, airplanes, pipes, steel bridges are also affected by acid rain. The irreplaceable damage caused to heritage buildings by acid rain is really a matter of concern. Effect on public health: It does not affect human health directly as the rainwater is too dilute to cause health issues. However, the pollutants like sulfur dioxide and nitrogen dioxide gases including their suspended particles like sulfates and nitrates reduce visibility that may lead to accidents and thus may cause injuries and deaths. Further, when it is in the form of inhalable fog, it causes health issues like eye irritation and asthma in humans as well as in animals. Effect on drinking water: It also causes corrosion of water pipes which leads to the leaching of heavy metals such as iron, lead and copper into drinking water. Real-life examples of damage caused by acid rain
How to control acid rainThe only way to reduce acid rain is by reducing the release of the pollutants that lead to it. Besides this, we can do our part by reducing our vehicle use and using public transport, walking, bicycle, carpooling, etc. The use of electricity can be reduced as it is produced using fossil fuels, also solar energy can be used instead. Types of acid rainThe acid rain or deposition can occur in two forms: wet and dry. Accordingly, acid rain can be of the following two types: 1. Wet Deposition:It occurs when the wind takes the acids present in the air to the wet regions and the acids fall to the ground in the form of rain, fog, mist, etc. These acids from the air are deposited on the earth's surface. This acid from the earth enters the drainage system, rivers and canals. 2. Dry deposition:If the wind carries the acids in the air to the regions with dry weather, the acidic pollutants mix with dust or smoke and fall to the ground as dry particles. It tends to stick to surfaces like houses, trees, cars and buildings.
Next TopicAcid Reflux
|
 For Videos Join Our Youtube Channel: Join Now
For Videos Join Our Youtube Channel: Join Now
Feedback
- Send your Feedback to [email protected]
Help Others, Please Share









