Oxalic AcidOxalic acid is an organic compound and a type of dicarboxylic acid. It is naturally found in many plants, leafy greens, vegetables and also in fruits, cocoa, nuts and seeds. It is odorless and colorless in solid-state, which can be dissolved in water. However, when it is purified, it changes into a white crystalline substance. Further, it is more acidic than oxalic acid. Further, oxalic acid is also produced in the human body when glyoxylic acid or ascorbic acid is metabolized and flushed out from the body through the urine. Also, being highly corrosive, it is more toxic as compared to other substances. So, it should be properly disposed of after usage. Oxalic Acid Chemical FormulaOxalic acid is an alpha, omega-dicarboxylic acid with the chemical or molecular formula C2H2O4 or (COOH) 2 or HOOCCOOH and molecular weight 90.03 g/mol. It is also called ethanedioic acid and is a conjugate acid of an oxalate (1- ) and an oxalate. It is the simplest dicarboxylic acid, which is made of two carboxylic acid groups (COOH) directly joined or bonded to each other at the carbon atoms, as shown in the below image. 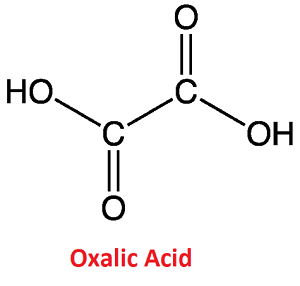
Oxalic acid in its anhydrous form or when it is dehydrated exists as two different polymorphs. Out of which, one has hydrogen bonding that helps develop the chain-like structure at the intermolecular level. Another polymorph also has hydrogen bonding but in this type, this bonding leads to the formation of a sheet-like structure at the intermolecular level. Physical properties of oxalic acid
Chemical properties of oxalic acid
Oxalic Acid UsesOxalic acid offers lots of uses. Some of its common and major uses are described below: Cleansing agent: It is widely used for cleaning the toughest stains. It is present in various cleaning products, bleaches, and detergents. It is also suitable for polishing stones and treating old wood. Industrial uses: In industries, it is used in the processing of minerals. It is also used to sterilize equipment and for bleaching purposes in the textile industry. Medicinal uses: In the pharma industry, it is also used to purity and dilute some chemicals. Reducing element: It is also used in developing photographic film. Water treatment: It is also required in the treatment of wastewater as it can easily remove calcium deposits from water. Rust: It is also used in a rust removal process wherein rust is removed from various iron products. Dye: It is also required in a process that is used to form dyes. Preparation of Oxalic AcidLaboratory preparation of oxalic acid:The preparation of oxalic acid is very easy. It is prepared by the oxidation of some carbohydrates by concentrated nitric acid. The steps are described below:
Industrial Preparation of Oxalic Acid
Health HazardOxalic acid is a strong acid with high toxicity. If it is ingested accidentally, it may cause severe symptoms like vomiting, diarrhea, renal damage, coma, shock, etc. It may also cause irritation in the eyes, skin, mucous membrane, and may also affect the kidney. The reason for the toxic nature of oxalic acid is that it forms calcium oxalate when it reacts with the calcium in the tissues. It disturbs the calcium and potassium ratio and may cause the accumulation of oxalates in the tubules of kidneys and thus may lead to kidney damage.
Next TopicTranexamic Acid
|
 For Videos Join Our Youtube Channel: Join Now
For Videos Join Our Youtube Channel: Join Now
Feedback
- Send your Feedback to [email protected]
Help Others, Please Share









