Formic AcidFormic acid is a fuming liquid. Its chemical formula is HCOOH. It is also known as methanoic acid (IUPAC name). It is the first member in a series of homologous carboxylic acids. It is found naturally in the venom of ants and bees and is an important intermediate in chemical synthesis. The itching or burning sensation that we feel when bit by wasps, honey bees, etc., is caused by the entry of formic acid into our body. Further, it is also present in small amounts in sweat, urine, and meat extract. Formic Acid Structure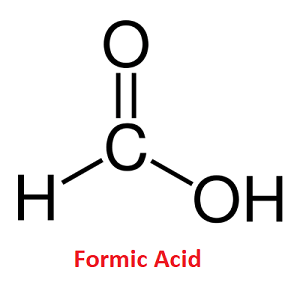
Formic acid has a simple structure wherein it has a single carbon atom that's why it is named methanoic acid. In its molecule, a carbon atom is bonded to hydrogen by a single bond and bonded to oxygen by a double bond and bonded to another oxygen atom by a single bond, this oxygen in turn is bonded with a hydrogen atom. In short, a single carboxylic acid group is bonded to a single hydrogen atom. Physical Properties of Formic Acid
Chemical Properties of Formic Acid
HCOOH + 2HgCl2 → Hg2Cl2 + 2HCl + CO2
HCOOH + PCl5 → HCOCl + POCl3 + HCl Preparation of Formic AcidIndustrial preparation:i) By the reaction of sodium formate and sulphuric acid: Sodium formate is produced from carbon monoxide and sodium hydroxide, the reaction takes place as follows; CO + NaOH + H2O → HCOONa (sodium formate) + H2SO4 → HCOOH (formic acid) ii) By the hydrolysis of methyl formate Methyl formate is formed when methanol combines with carbon monoxide in the presence of a strong base, as shown below; CH3OH + CO → HCO2CH3 (Methyl formate) The methyl formate undergo hydrolysis to form formic acid as shown below; HCO2CH3 + H2O → HCOOH + CH3OH iii) By the reaction of formamide with sulphuric acid In some cases, methyl formate is treated with ammonia to form formamide, which is later hydrolysed with sulphuric acid to form formic acid as shown below: HCO2CH3 + NH3 →HC(O)NH2 + CH3OH 2HC(O)NH2 + 2H2O + H2SO4 → 2HCO2H + (NH4)2SO4 Laboratory MethodIn this method, it is prepared from glycerol and oxalic acid. The steps are described below: i) Add 40 gm of oxalic acid crystals and 50 ml of anhydrous glycerol in a distillation flask. ii) Heat the flask slowly at 100 degrees Celsius to 110 degrees Celsius using a sand boiler. iii) The aqueous solution of formic acid is distilled and then collected in the receptor. The glycerol is left behind in the distillation flask. So, formic acid can be prepared again by adding oxalic acid to the distillation flask and heating it to the required temperature. The reactions involved in the above method are as follows; i) Glycerol and oxalic acid react to form glycerol mono oxalate. ii) When glycerol mono oxalate is heated to 100 degrees Celsius, one carbon dioxide molecule separates and it is converted into additive glycerol monoformate. iii) Glycerol monoformate reacts with water produced in the first step of this reaction, and oxalic acid crystals to form Formic acid and glycerol. Uses of Formic Acid
Health HazardsDilute formic acid is not toxic, it acts as a food additive. But, in concentrated form, it is highly corrosive and harmful. For example, if someone happens to inhale its fumes, it may cause irritation of the mucous membrane and may also cause burns and blisters on the skin. Further, exposure to formic acid for a long time may damage the kidney.
Next TopicLewis Acids and Bases
|
 For Videos Join Our Youtube Channel: Join Now
For Videos Join Our Youtube Channel: Join Now
Feedback
- Send your Feedback to [email protected]
Help Others, Please Share









